He proposed that an atom consists of a uniform sphere of positive electricity in which the electrons are distributed more or less uniformly. A small percentage of particles were deflected through large angles.
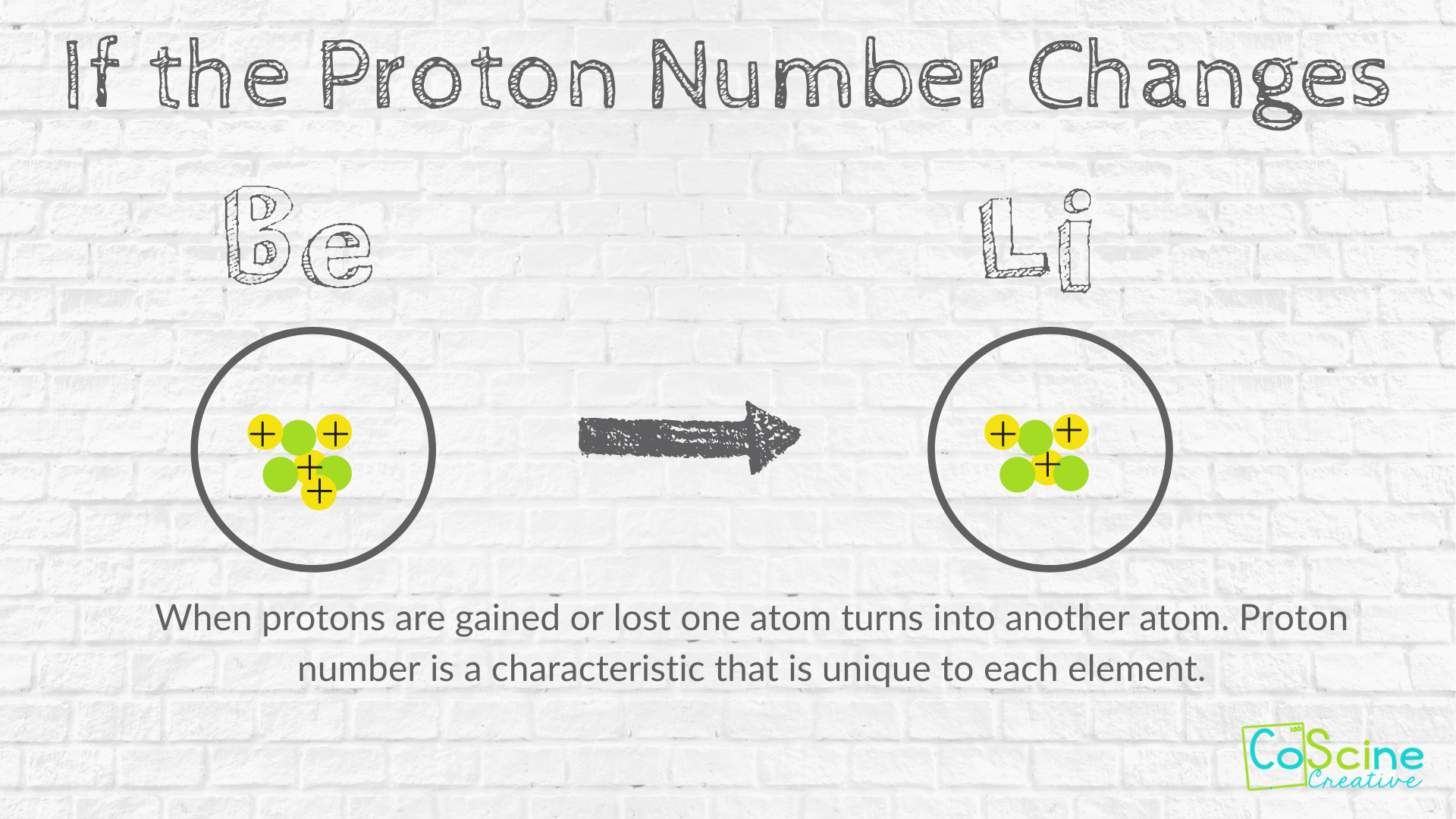
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative
Over the last 100 years scientists have done investigations which show that atoms are made up of even smaller particles.

. Describe how the model of the atom has changed over time. There are three types of particles in an atom. Doesnt alter its chemical identity.
State the kind of change in matter in each example below. His model was circular smaller unit of matter His model was inaccurate because. The postulate of Daltons atomic theory that atom is indivisible particle and can be subdivided into smaller particles was later changed because atom can be divided into neutrons protons and electrons.
Rutherfords experiment prompted a change in the atomic model. View the full answer. According to John dalton atomic theory atom is considered smallest unit which cannot be divided further.
Neutrons Neutrons do not have a charge. Describe Rutherfords model of the atom including the location of protons neutrons. Scientist now know that atoms are made up of electron which have a ___ charge.
Dalton theorized that atoms are indivisible but the discovery of ___ particles changed this theory. The neutron was discovered in 1932. Sarawatlism sarawatlism 12042020 Chemistry High School answered How does the number of.
Protons are positive neutron are neutral having no charge and electrons are negative. Majority of the particles were not deflected at all. Get started for FREE Continue.
Thomsons model of an atom Thomson was the first to propose a detailed model of the atom. During chemical changes particles do change with atoms or ions regrouping. In respect to this how was the atomic model developed.
Previous question Next question. If the positive alpha particles mostly passed through the foil but some bounced back. In 1913 Neils Bohr a student of Rutherford s developed a new model of the atom.
Protons neutrons and electrons. During chemical reactions atoms rearrange to make different substances This atomic model has changed over time. Occurring in the nucleus of the atom when it emits or absorbs rays or particles when the nucleus splits or joins with another.
And ___ which are neutral. Resulting from the breaking of bonds between atoms to form pure substances different from the organized substances. In your answer include the work of Thomson Rutherford and Bohr and the Geiger-Marsden experiment.
This model was known as the plum pudding model. The neutron has about the same mass as the proton. Electrons of different atoms come together to participate in chemical bonding.
Electrons are the subatomic particles that revolve around the nucleus of an atom. Click here to get an answer to your question How does the number of subatomic particles change as the carbon isotope decays. Thus the atom as a whole is electrically neutral.
But later discovery of electron proton and neutron lead our knowledge further and now we know that atom is made up of these particles thu. The negative and the positive charge are equal in magnitude. ___ which have a positive charge.
A few points detailing the discovery and the properties of electrons are listed below. In other words they are neutral. Bonds links between atoms break and new ones form and energy is either given out or taken in.
AND if they already knew that the electron was. The first model of the atom was developed by JJ Thomson in 1904 who thought that atoms were composed purely of negatively charged electrons. The historical development of atomic models.
During chemical changes particles do change with atoms or ions regrouping. Thomson discovered the electron a negatively-charged particle. The atom was imagined as a small indivisible ball similar to a very tiny ball.
Oct 11 2014. An atoms net charge is determined by comparing the number of protons and electrons that are in each atom. How the Model of the Atom has changed over Time John Dalton Neils Bohr Made his discovery in 1800 His model was accurate because he joined wooden balls together to show that atoms form compounds.
A small proportion of particles were deflected by the dense positive charge of the nucleus. These electrons may be removed from or gained by an atom to form ions. Sometimes the results of.
The postulate of Daltons atomic theory that atom is indivisible particle and can be subdivided into smaller particles was later changed because atom can be divided into neutrons protons and electrons. Scientists used the model to make predictions. There is no universal net charge for atoms.
Describe how and why the atomic model has changed over time. Bonds links between atoms break and new ones form and energy is either given out or taken in. This theory was then disproved by Ernest Rutherford and the gold foil experiment in 1911 where Rutherford shot alpha particles at gold foil and noticed that.

How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative
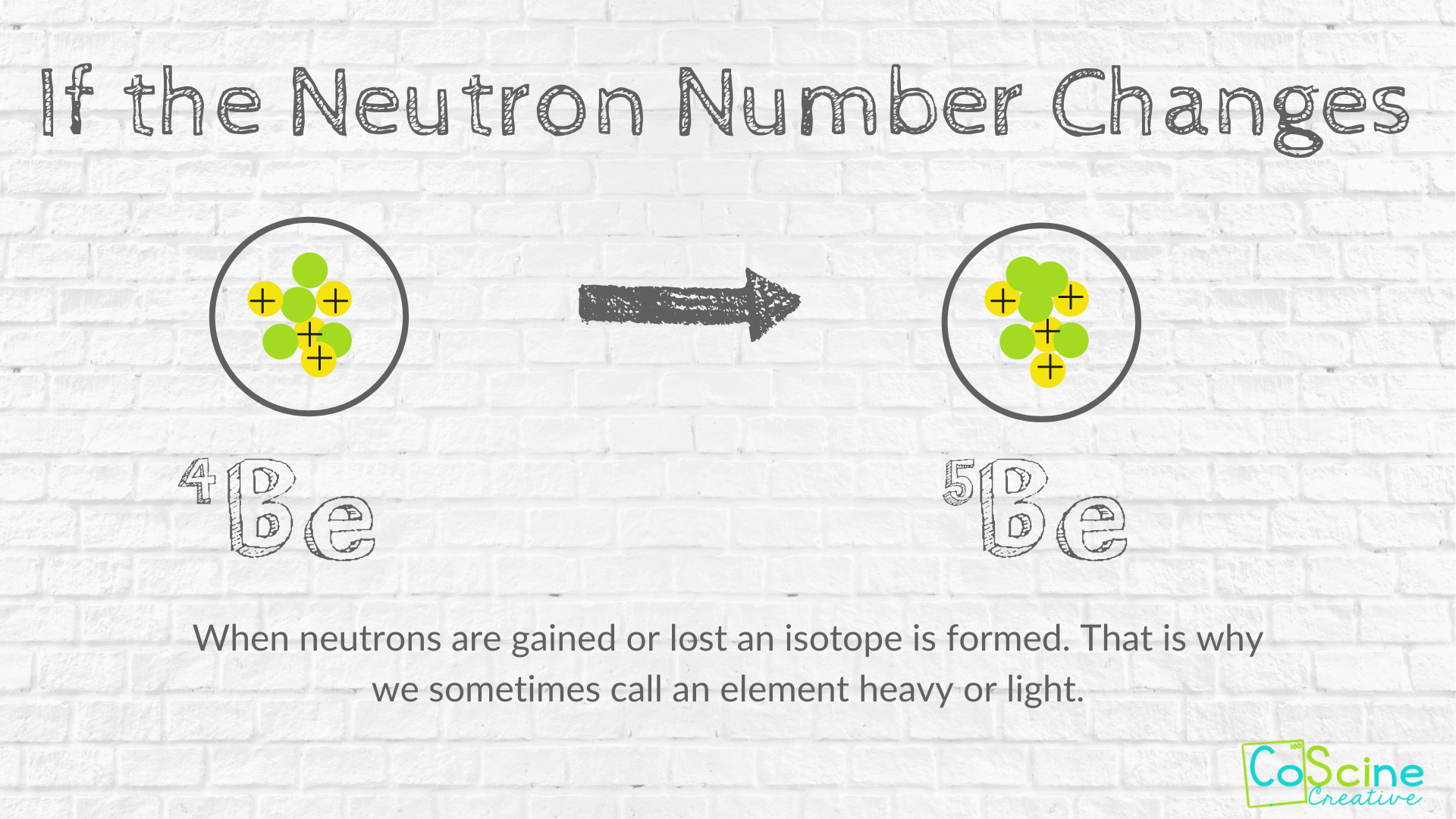
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative

Other They Exchange Gluons And Create A Strong Color Force Field That Binds Quarks Together The Force Field Ge Atomic Theory Teaching Chemistry Learn Physics
0 Comments